Photocrosslinking
Commercial trifunctional probes
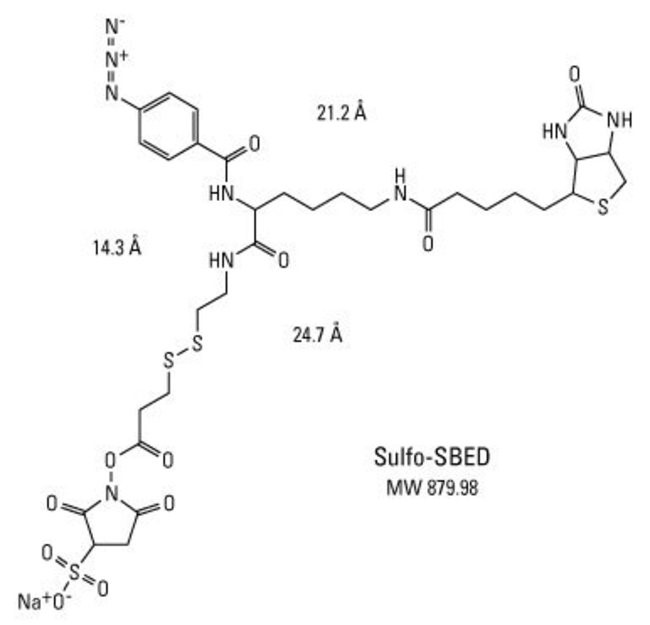
Membrane-protein interactions
Membrane-protein interactions are difficult to study because when the membrane is stripped away, their shape and behaviour changes; they may not link with their typical partners.
UAAs containing photocrosslinker side chains
To site-specifically incorporate UAAs into proteins, an orthogonal aminoacyl-tRNA synthetase (aaRS) that charges the UAA onto its cognate tRNA is used. This allows the specific incorporation of an UAA during mRNA translation in response to an amber stop codon (amber suppression) placed at a user-defined site in a gene of interest
Most widely used orthogonal synthetase/tRNA pairs:
Methanocaldococcus jannaschi Tyrosyl tRNA synthetase (MjTyrRS)/tRNACUA pair —— orthogonal in E. coli.
Pyrrolysyl tRNA synthetase/tRNA CUA pairs from Methanosarcina mazei (Mm) and Methanosarcina barkeri (Mb) ——orthogonal to endogenous aaRS/tRNA pairs in E. coli and eukaryotic cells, including multicellular organisms.
Hitherto more than 250 UAAs can be incorporated site-specifically into prokaryotic and/or eukaryotic cells using engineered MjTyrRS or PylRS mutants. (Angew. Chem. Int. Ed. 2018, 57, 14350 – 14361 )
Requirements:
Chemically stable.
Bioorthogonal to all functional groups in the cell prior to excitation with the appropriate wavelength of light.
excitation should be possible at longer wavelengths that are not harmful to biomolecules and living cells.
Small size of the photocrosslinker to minimally perturb natural binding properties.
High and specific crosslinking efficiencies to generate stable adducts.
[upl-image-preview uub0aad7e-0d2f-4831-8cc8-65ec5c11930b url=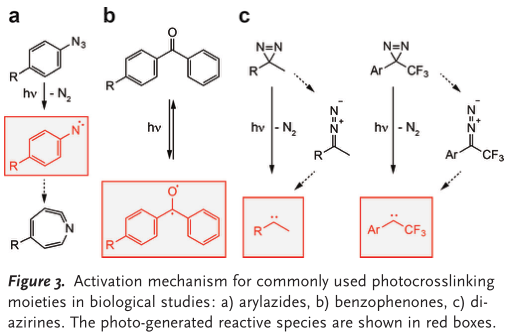 alt={TEXT?}]
alt={TEXT?}]
| Reactive intermediate | irradiation | nearby | life time | |
|---|
| Aryl azides | nitrene species | 250 nm | C—H and heteroatom— H bonds. | 100 μs | |
| Benzophenones | ketyl diradicals | 350–365 nm | C—H bonds (preferencefor the γ-CH bond of methionine) | 120 μs | bulkiness, hydrophobic core, rigidity, and potential sidereactivity with amines |
| Diazirines | carbene | 350–365 nm | C−H and heteroatom−H bonds of molecules in close proximity | in the nanosecond range | small size.Crosslinking efficiency may be reduced by quenching of the carbene intermediate with water or by rearrangement to a more stable diazo isomer that reacts with nucleophiles and may increase the incidence of nonspecific crosslinking events. |
Functionalization of the diazirine moiety with trifluoromethyl and aromatic groups has been reported to increase formation of the reactive carbene intermediate while stabilizing the diazo isomer, thereby leading to reduced non-specific crosslinking with nucleophiles. (J. Biol. Chem. 1980, 255, 3313 – 3318)

2002. P. G. Schultz. PNAS
10.1073/pnas.172226299
Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli.
The first photocrosslinking amino acid incorporated into proteins in E. coli
2013. Lei Wang. Cell
10.1016/j.cell.2013.11.008
Genetically Encoded Chemical Probes in Cells Reveal the Binding Path of Urocortin-I to CRF Class B GPCR.
[upl-image-preview uud432f8b-9002-4e4e-a74d-ae155543f6dd url= alt={TEXT?}]
alt={TEXT?}]
Coin et al. addressed the activation mechanism of class B GPCRs upon interaction with peptide hormones in mammalian cells using UAAs.21
Azide‐bearing amino acid 2 was incorporated at different positions in the transmembrane domain of the class B corticotropin releasing factor receptor type 1 (CRF1R) in HEK293 cells.
UV irradiation in the presence of the native ligand urocortin‐I revealed the ligand‐interaction area within the receptor. Subsequent pairwise chemical crosslinking using UAA 14 (see Section 3.1, Figure 7) incorporated site‐specifically into the receptor and cysteine mutants of the urocortin ligand allowed conclusions to be drawn on the binding orientation.
2016. Strømgaard. Nat Commun
Rannversson et al. mapped the binding sites for two antidepressants (imipramine and vortioxetine) on the human serotonin receptor (hSERT).
hSERT regulates neuronal signalling by orchestrating serotonin metabolism. Inhibitors of hSERT are extensively used in the treatment of psychiatric diseases, such as depression and anxiety.
In many cases, however, the exact binding mode to the human receptor is not known in detail.
To elucidate drug‐binding areas on the receptor, UAA 2 was incorporated at 75 different positions within hSERT in living mammalian cells.
UV‐induced crosslinking unequivocally revealed binding of both drugs to the central substrate‐binding site of hSERT.

2005. Yokoyama. Nat Methods.
10.1038/nmeth739
Protein photo-cross-linking in mammalian cells by site-specific incorporation of a photoreactive amino acid.
Hino et al. conducted intensive photocrosslinking and MS studies in mammalian cells to dissect downstream signalling cascades upon receptor activation.23
Upon EGFR activation, the adaptor protein Grb2 mediates the transduction of external stimuli to intracellular proteins such as Ras, thereby inducing further downstream signalling events.
Site‐specific incorporation of UAA 1 into Grb2 in live mammalian cells and subsequent UV irradiation revealed that Grb2 is recruited to EGFR upon receptor stimulation, but not in the resting state.23b
Furthermore, genetic encoding of 3 into the Src homology 2 (SH2) domain of Grb2 in living mammalian cells followed by UV irradiation after an EGFR stimulus, revealed and identified, in combination with comparative MS analyses, new SH2‐domain binders.
2011. Yokoyama*, Sakamoto*. J. Mol. Biol.
10.1016/j.jmb.2010.12.022
Genetic Incorporation of a Photo-Crosslinkable Amino Acid Reveals Novel Protein Complexes with GRB2 in Mammalian Cells.
Selenium‐based photocrosslinker UAAs
P.R. Chen*, 2014. JACS
https://doi.org/10.1021/ja504371w
Genetically Encoded Cleavable Protein Photo-Cross-Linker.
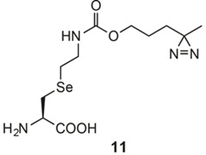
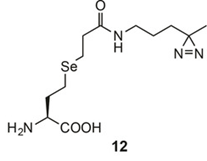
P.R. Chen*, 2016. Nat. Commun.
10.1038/ncomms12299
Genetically encoded protein photocrosslinker with a transferable mass spectrometry-identifiable label.
P.R. Chen*, 2017. Angew
10.1002/anie.201708151
Quantitative and Comparative Profiling of Protease Substrates through a Genetically Encoded Multifunctional Photocrosslinker.

2017. A.K. Mapp. ChemBioChem
10.1002/cbic.201600578
A Bifunctional Amino Acid Enables Both Covalent Chemical Capture and Isolation of in Vivo Protein–Protein Interactions.

isolation and visualization of the crosslinked yeast Gal4/Gal80 complex from whole‐cell lysate.