UAAs with electrophilic moieties that undergo proximity‐enabled crosslinking that can be incorporated site‐specifically into proteins
2013. Lei Wang. Nat Method
Adding an unnatural covalent bond to proteins through proximity-enhanced bioreactivity.
10.1038/nmeth.2595
[upl-image-preview uu1501619-abef-4d06-aecf-a1830e6d751b url=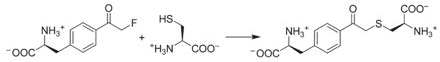 alt={TEXT?}]
alt={TEXT?}]
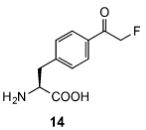
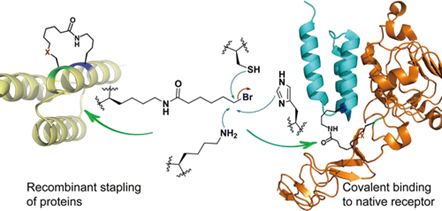
The first successful application.
The fluoroacetamide moiety reacted in a SN2 reaction with nearby cysteines when incorporated site‐specifically into proteins.
Lei Wang*. 2014 ACS Chem. Biol.
Genetically Encoding an Electrophilic Amino Acid for Protein Stapling and Covalent Binding to Native Receptors.
10.1021/cb500453a
Since cysteines are not commonly found on protein surfaces, proximity‐triggered interprotein crosslinking using these UAAs is dependent on genetic introduction of cysteine residues in a protein of interest.
To target other nucleophilic natural amino acids as well, an UAA bearing a linear, flexible bromoalkyl chain (17, n=5) has been developed.
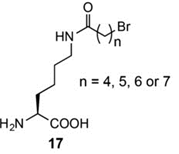
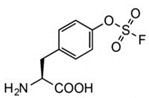
Target diversity: Cys, His or Lys
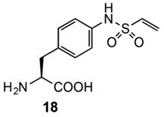
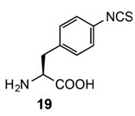
To expand chemical crosslinking reactivity toward other nucleophilic amino acid residues besides cysteines, further UAAs bearing vinylsulfonamide (18), aryl isothiocyanate (19), aryl carbamate (20), and aryl fluorosulfate (21) moieties were designed.

they often require basic conditions (pH >8.5), which restricts their application to in vitro protein crosslinking experiments. (P. G. Schultz*, 2017, Angew)
Some of these amino acids, however, showed side reactions with glutathione upon incorporation into proteins. (Kim*, 2014. JACS)
Requirement:
finely tuning the reactivity of the electrophilic moieties of chemical crosslinker UAAs to be inert towards abundant endogenous nucleophiles and
to react with nucleophilic amino acid side chains only in a proximity‐dependent manner.
Kim*, 2014. JACS
10.1021/ja502851h
A Genetically Encoded aza-Michael Acceptor for Covalent Cross-Linking of Protein–Receptor Complexes.
P. G. Schultz*, 2016, Angew
P. G. Schultz*, 2017, Angew
10.1002/anie.201611841
Protein Crosslinking by Genetically Encoded Noncanonical Amino Acids with Reactive Aryl Carbamate Side Chains.
[upl-image-preview uu7e742cc-79a4-45b5-8fa7-2d57d9bf4337 url=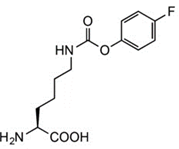 alt={TEXT?}]
alt={TEXT?}]
Lei Wang*. 2018, JACS
10.1021/jacs.8b01087
Genetically Encoding Fluorosulfate-l-tyrosine To React with Lysine, Histidine, and Tyrosine via SuFEx in Proteins in Vivo.